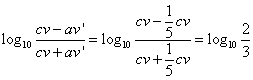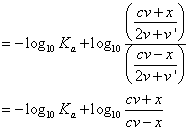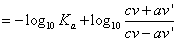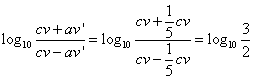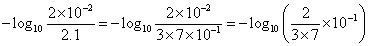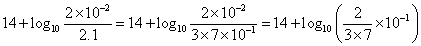 Bという反応を考える。
Bという反応を考える。
「正反応」の反応速度v[mol/s]は、
| v = - | d[A] | ・・・(1) |
| dt |
もっとも単純な場合として、上の「正反応」の反応速度が反応物Aの濃度に比例している場合を考える。すなわち、
v=k[A] ・・・(2)
(1)(2)より、反応物Aの濃度[A]を、時間tの関数として、C(t)と書くと、
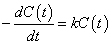
この「微分方程式」は、
tの関数C(t)を1階微分すると、元の関数C(t)に比例するが、符号は逆になる、
ということを意味している。このような性質を満たす関数を探すのが、「微分方程式」を解く、という作業である。
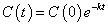
時刻t=0における濃度をC(0)として、このような関数が得られる。
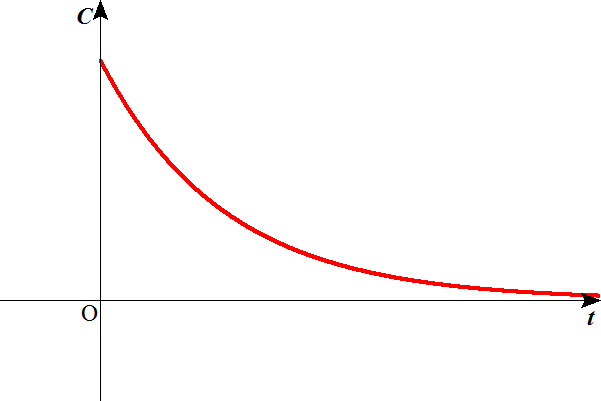
底が1より小さい指数関数であるから、全区間単調減少である。
放射性同位元素が崩壊する反応、有機化合物の一方の異性体が他の異性体に変化する反応など、この形式をとるものが実際に存在する。
反応速度が反応物の濃度の1乗に比例するから、「1次反応」と呼ぶ。
これは、反応物の粒子が、他の粒子の影響を受けず、単独で自発的に反応を起こす場合であると考えられる。
「箱の中にn個の赤玉がある。ここから一つ選ぶ方法の数は、nC1=n」、まさに粒子の個数に比例している(!)というわけだ。
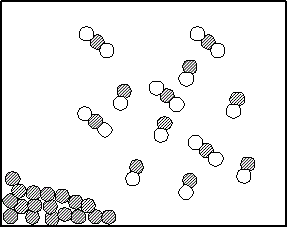
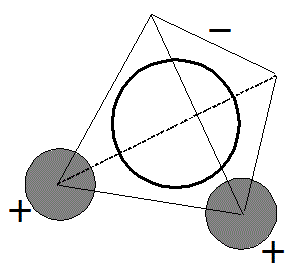
 Cという反応の「正反応」では、AとBの粒子同士が、出会わないことには、反応が始まらない。
Cという反応の「正反応」では、AとBの粒子同士が、出会わないことには、反応が始まらない。
単純化すると、出会う確率は、反応物A,Bの濃度[A],[B]のいずれにも比例する、といえないだろうか?
「箱の中にm個の白球とn個の赤玉がある。ここから白玉と赤玉のペアを一組選ぶ方法の数は、mn通り」、ではないか!
v=k[A][B]
で反応速度が表されるものを、濃度の積(2次式)に比例する、という意味で「2次反応」という。
 Bという反応物が1種類である反応にも「2次反応」は考えられる。
Bという反応物が1種類である反応にも「2次反応」は考えられる。
v=k[A]2
と表される場合だ。これは、
「箱の中にn個の赤玉がある。ここから二つ選ぶ方法の数は、nC2=
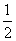 n(n-1)通り」、
n(n-1)通り」、
そして、粒子の数が充分大きければ、n
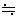 n-1、近似的に個数の2乗に比例している、と考えればつじつまは合う。
n-1、近似的に個数の2乗に比例している、と考えればつじつまは合う。
この反応は、上の「1次反応」の場合と異なって、同一種類の粒子が、互いに衝突することではじめて反応が起こる場合、と考えられる。
aA+bB
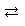 cC+dDという反応の「正反応」の反応速度vは、
cC+dDという反応の「正反応」の反応速度vは、
v=k[A]a[B]b
と、書くことができそうにも思える。ところが、現実は、そんなに単純ではない。実際には、
aA+bB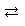 cC+dDという反応の「正反応」の反応速度vは、 cC+dDという反応の「正反応」の反応速度vは、
v=k[A]a'[B]b' ただし、a',b'は、実験によって定める。 |
化学反応式、というのは、現実に次々に生じている複雑な数々の多段階の反応について、途中経過を無視して、最初と最後の、収支決算だけを表している。
たとえば、A
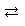 Bという反応も、
Bという反応も、
A
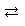 C v1=k1[A]
C v1=k1[A]
C
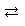 D v2=k2[C]
D v2=k2[C]
D
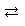 B v3=k3[D]
B v3=k3[D]
という3段階の「素反応」からできていて、それそれの「素反応」は、単純な「1次反応」であったかもしれない。
しかし、全体として、A
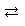 Bという反応の反応速度は、それらの中でもっとも遅い反応(「律速段階」という)によって決まることになる。
Bという反応の反応速度は、それらの中でもっとも遅い反応(「律速段階」という)によって決まることになる。
上の例でたとえば、第2段階の反応が最も時間がかかるのだとすれば、A
 B全体の反応速度は、v2に近いものになり、必ずしもAの濃度に比例するとはいえなくなるのも当然であろう。
B全体の反応速度は、v2に近いものになり、必ずしもAの濃度に比例するとはいえなくなるのも当然であろう。
N2+3H2
 2NH3
2NH3
「正反応」が起こるとき、窒素分子1個と水素分子3個が同時に衝突する(!)、などということが、実際に起こっているとは、考えられないのである。
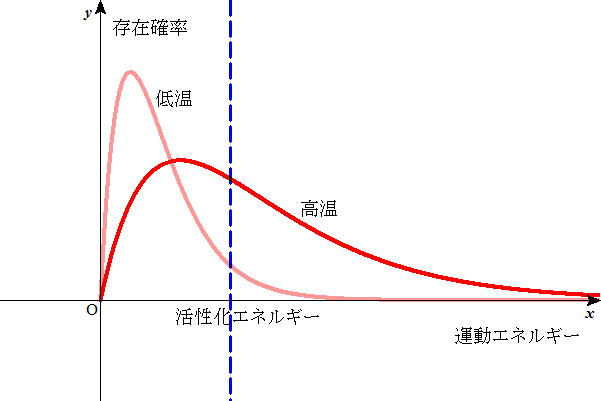
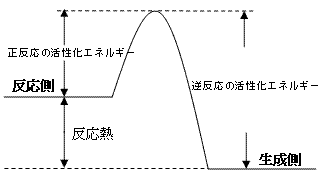
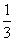 の3乗で、27分の1。
の3乗で、27分の1。


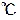 における酢酸の「電離平衡定数」Kaは、2.75×10-5、大雑把に
Ka=3×10-5
として、log103
における酢酸の「電離平衡定数」Kaは、2.75×10-5、大雑把に
Ka=3×10-5
として、log103