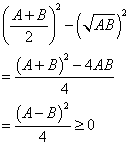次式で定義される酢酸の電離平衡定数Kaの値を3×10-5とする。
| Ka= | [CH3COO-][H+] |
  | |
| [CH3COOH] |
|
【問題】pH3に調整した酢酸1Lと、pH5に調整した酢酸1Lとを混合した溶液のpHは、どうなるだろうか?
次式で定義される酢酸の電離平衡定数Kaの値を3×10-5とする。
|
| CH3COOH | CH3COO- | + | H+ | ||
    | |||||
| はじめ | x | ||||
| 反応・生成 | xα | xα | xα | ||
    | |||||
| 電離平衡後 | x(1-α) | xα | xα | ||
| Ka= | [CH3COO-][H+] | = | (xα)2 | = | xα2 |
  |  |  | |||
| [CH3COOH] | x(1-α) | 1-α |

|
α=3(1-α) α=3/4=0.75 x=4/3×10-5 |
|
α=0.03(1-α) 100α=3(1-α) α=3/103 x=103/3×10-3 |
| CH3COOH | CH3COO- | + | H+ | ||
    | |||||
| はじめ | x1+x2 | ||||
| 反応・生成 | (x1+x2)α | (x1+x2)α | (x1+x2)α | ||
    | |||||
| 電離平衡後 | (x1+x2)(1-α) | (x1+x2)α | (x1+x2)α | ||
| Ka= | [CH3COO-][H+] | = | [{(x1+x2)α}/2]2 | = | (x1+x2)α2 |
  |   |  | |||
| [CH3COOH] | (x1+x2)(1-α)/2 | 2(1-α) |
|
1140α2+2α-2=0
0 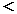 α α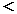 1だから 1だから
α=4.10×10-2 |
| pH=-log10 | A+B | =-log10 | 10-5+10-3 | =-log10 | 10-5(100+1) |
 |  |  | |||
| 2 | 2 | 2 |
| pH'= | 5+3 | = | -log10A-log10B | = | -log10AB | =-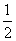 log10AB= log10AB=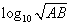 |
 |   |  | ||||
| 2 | 2 | 2 |